Isothermal Titration Calorimeter
MicroCal PEAQ-ITC, Malvern Panalytical

Isothermal titration calorimetry (ITC) is a analytical technique capable of characterizing interactions between biomolecules in solution. ITC directly measures the heat released or absorbed during a binding event to accurately determine a wide range of thermodynamic parameters, including binding affinity (KD), enthalpy (ΔH), entropy (ΔS), and reaction stoichiometry. These measurements can be made in a single experiment and do not require labeling or immobilization of the biomolecules.
In an ITC experiment, ligand (or macromolecule) solution is gradually titrated into a cell containing a macromolecule solution at constant temperature. As the two molecules interact, heat is released or absorbed in direct proportion to the amount of binding, which is detected and measured by the calorimeter. As the macromolecule becomes saturated with ligand, less binding occurs and less heat is released/absorbed. Integration of the heats are used to extract thermodynamic parameters.
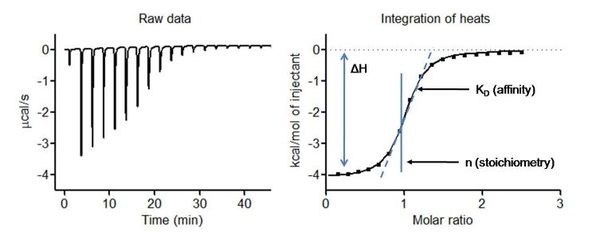 An example titration of CaCl2 into EDTA
An example titration of CaCl2 into EDTA
Features
- No labeling or immobilization of biomolecule required
- Determination of binding affinity (KD), enthalpy (ΔH), entropy (ΔS), and reaction stoichiometry in a single experiment
- Measure heat capacity of binding by performing multiple titration experiments at varying temperatures
- Directly measure millimolar to nanomolar dissociation constants (10-2 to 10-9 M)
- Measure nanomolar to picomolar dissociation constants (10-9 to 10-12 M) through competitive binding experiments
- No limits on macromolecule or ligand size
- Experiments are computer controlled allowing unattended operation after set-up
- Automated washing of sample cell and syringe
- Temperature control: 2°C – 80°C
- Sample cell volume is 200 µL and syringe volume is 40 µL
Applications
- Drug discovery:
- Hit validation and characterization
- Optimization of candidates
- Mechanism of action
- Quantitative measurement of interaction between any two biomolecules (protein, RNA, DNA, lipids, drugs and inhibitors):
- Confirm binding and activity
- Determine thermodynamic parameters and stoichiometry
- Assessing effect of molecular structure changes on binding mechanisms
- Analysis of enzyme kinetics
Internal Rates
- Unassisted: $25.00/hr
- Assisted: $76.00/hr